Introduction
Given the current challenges with — in some countries bans of — Glyphosate, Glufosinate has become a possible candidate to partially replace the widespread usage of Glyphosate in agriculture. Glufosinate, however, is long plagued by three major issues — the high cost (roughly 2x-3x over Glyphosate), high environmental impact, and relatively small selection of Glufosinate-resistant seeds. L-glufosinate is the next generation, more environmentally friendly replacement of Glufosinate. But manufacturing L-glufosinate at a low cost and with scale is still a challenge. Currently, manufacturers synthesize L-glufosinate primarily in two ways: through asymmetric chemical synthesis and relying on biochemical processes. Both methods have serious constraints on volume and cost.
To improve the market competitiveness of Glufosinate, Vulpes developed a number of different, patent-pending manufacturing processes for L-glufosinate, to reduce Glufosinate’s environmental impact, reduce its cost, and potentially better place Glufosinate as a possible candidate to replace Glyphosate. Vulpes’s innovation is possible because Vulpes’s team has 20 years of history working on chiral amino acids and their derivatives. For example, its technology on chiral Aminobutyric acids and their derivatives upset the global market of Levetiracetam, an anti-seizure drug. 20 years later, Vulpes’s team’s innovation is still the main manufacturing approach for Levetiracetam.
Vulpes’s Innovation
Across Vulpes’s multiple manufacturing processes for L-glufosinate, Vulpes is able to achieve a standard, shared set of characteristics:
- Environment-friendly and simple process
- Globally competitive cost
- Piloted and sample ready
- 99%+ L-isomer ratio
- 98%+ L-glufosinate purity
In Vulpes’s “conversion route,” Vulpes has developed more than one simple, one-step processes to convert racemic Glufosinate into L-glufosinate at up to 85% of yield with no specialty equipment needed.
Sample COA
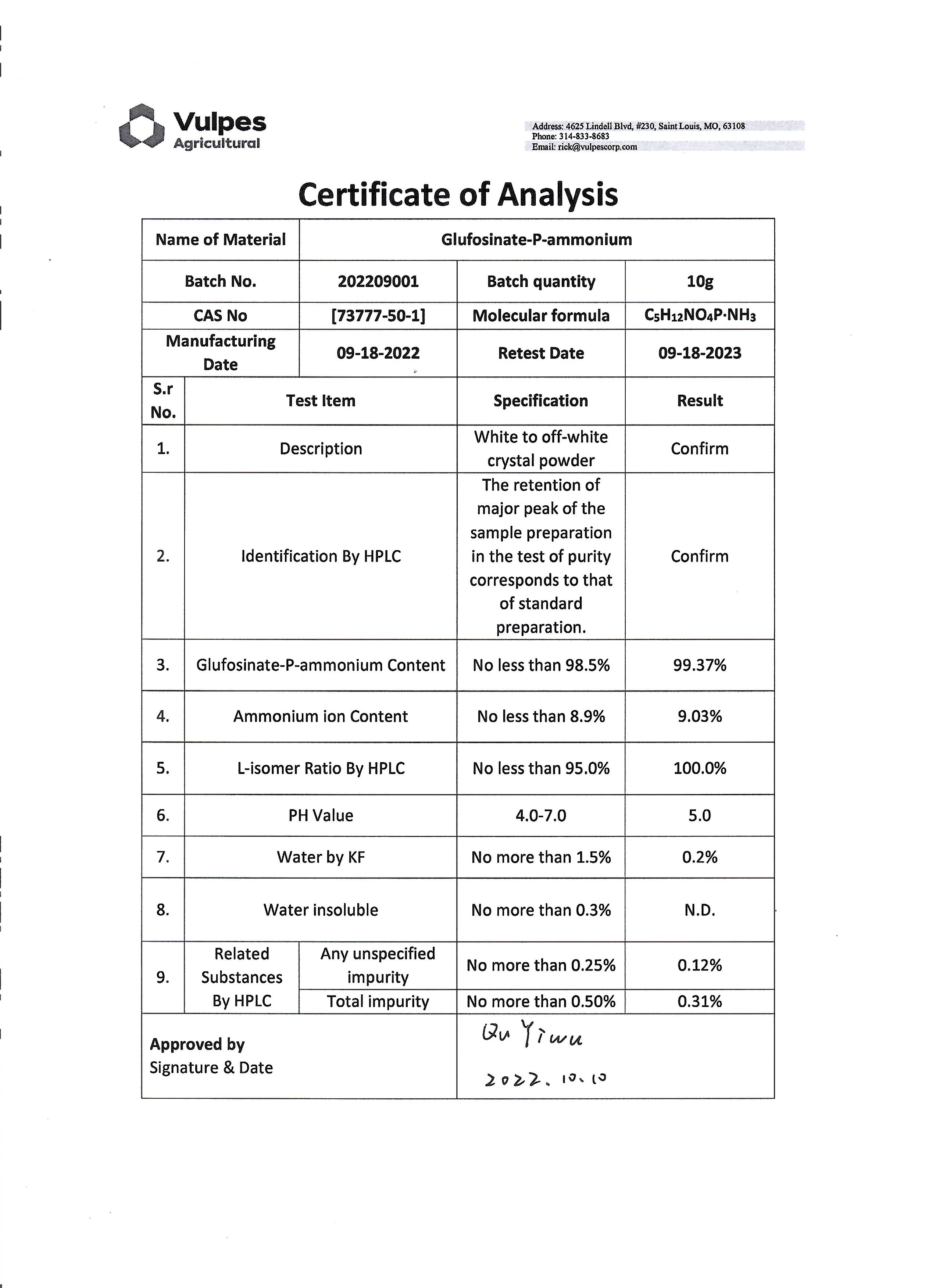
If you are interested in acquiring a sample or seeking partnership, please contact us at [email protected] or at (314) 833-8683
